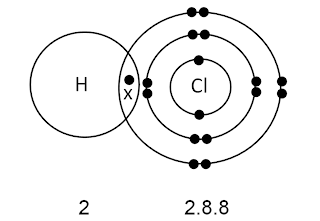
Formation of hydrogen chloride molecule, HCl ( covalent
compound)
The formation of covalent bonds in hydrogen chloride molecule is explained as follows:
§
Each hydrogen atom
has an electron arrangement of 1.
§ The outermost shell needs one electron in order to achieve
a stable duplet electron arrangement.
§ Clorine
atom has an electron arrangement of 2.8.7.
§ The outermost shell needs one electron in order to
achieve a stable octet electron arrangement.
§ Clorine
atom contributes one valence electron
while hydrogen
atom contributes one valence electron for sharing.
§ A single covalent
bond is formed.
Remarks:
§ The electrons must be drawn in the overlap of two
shells.
§ Students may use Lewis structure in drawing the
formation of covalent compound. ( Lewis structure only show the valence
electrons)
No comments:
Post a Comment